Addition of growth factors to unique system helps new bone formation
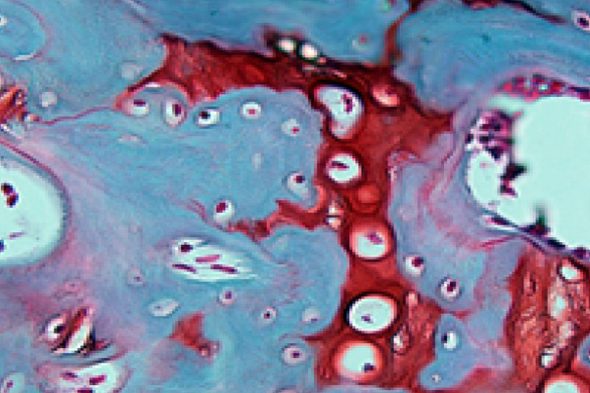
The development of new bone can be a multistep process: first, stem cells differentiate into cartilage cells. Next, the cartilage cells become bone cells. But that’s not all: the cells must experience some mechanical stresses during the transformation in order to transform efficiently from stem cells to bone cells.
Researchers at the University of Illinois at Chicago, in collaboration with colleagues at the University of Pennsylvania and Case Western University, have been working on a unique system that delivers stem cells to sites of bone defects and uses flexible fixators — pins and rods that are used to hold bones together — to provide tunable amounts of mechanical stress.
Now, these researchers, led by Eben Alsberg, the Richard and Loan Hill Professor of Bioengineering and Orthopaedics in the UIC College of Engineering, and Joel Boerckel, assistant professor of orthopaedic surgery at the University of Pennsylvania, have come up with a way to provide timed release of two growth factors that mimic the bone formation process that occurs during embryonic bone development in a rat model.
Together, the stem cells, flexible fixators and timed release of growth factors could one day help heal large bone defects or breaks in people. Their results are published in the journal Science Advances.
“What we are doing, in effect, is delivering the right growth factors at the right time to encourage the development of bone from stem cells in the same way that it happens during natural bone healing and development,” said Alsberg, who is a corresponding author on the paper along with Boerckel.
In previous work, Alsberg’s group at UIC and colleagues at the University of Pennsylvania developed the flexible fixators and stem cell “condensates,” which are masses of stem cells connected to one another so that they can be moved as sheets or plugs. The condensates allow the stem cells to be placed in specific areas of the body, such as within bone gaps or defects without the risk of floating away, as is often the case when stem cells are delivered to sites in the body through an injection of cell-containing liquids. When used together, the condensates and flexible fixators allowed for enhanced healing of bone defects in a rat model.
In the current research, Alsberg and colleagues added another layer to their system: they incorporated multiple growth factors into the condensates — one that helps transform stem cells into cartilage cells called transforming growth factor beta1 of TGF-beta1, and another that promotes the transition of this cartilage into bone called bone morphogenic protein 2 or BMP-2.
In rats with bone gaps in their femurs, the application of Alsberg’s system, including the growth factors, helps encourage the growth of new bone with enhanced function at 12 weeks compared with rats where growth factors weren’t included or only a single growth factor was included in the condensate/flexible fixator system.
“The bone formation achieved was comparable to that which occurs when BMP-2 is soaked in a collagen sponge and applied to bone breaks, which is currently an FDA-approved tissue engineering product for spinal fusion,” Alsberg said. “The collagen product can result in bone-forming where it’s not wanted, but in our system, bone only formed in the areas where we placed the growth factor-infused condensates.”
Samuel Herberg, Phuong Dang, Danial Alt, Rui Tang, Daniel Varghai, Jung-Youn Shin, Alex McMillan, Anna Dikina, Felicia He, Yu Bin Lee, Yuxuan Cheng, Kentaro Umemori and Honghyun Park of Case Western University, Pei-Chun Wong of Taipei Medical University and Anna McDermott, James Dewahare, and Joel Boerckel of the University of Pennsylvania, are co-authors on the paper.
Funding for this work was provided from the National Institutes of Health’s National Institute of Dental and Craniofacial Research (F32DE024712), National Institute of Arthritis and Musculoskeletal and Skin Diseases (R01AR066193, R01AR063194, R01AR069564), National Heart, Lung and Blood Institute (T32HL134622), and National Institute of Biomedical Imaging & Bioengineering (R01EB023907), the Ohio Biomedical Research Commercialization Program under award number TECG20150782, the Naughton Foundation and the Indiana Clinical and Translational Sciences Institute, Grant Number UL1TR001108 from the National Institutes of Health.
