Stackable artificial leaf uses less power than lightbulb to capture 100 times more carbon than other systems

Engineers at the University of Illinois Chicago have built a cost-effective artificial leaf that can capture carbon dioxide at rates 100 times better than current systems. Unlike other carbon capture systems, which work in labs with pure carbon dioxide from pressurized tanks, this artificial leaf works in the real world. It captures carbon dioxide from more diluted sources, like air and flue gas produced by coal-fired power plants, and releases it for use as fuel and other materials.
“Our artificial leaf system can be deployed outside the lab, where it has the potential to play a significant role in reducing greenhouse gases in the atmosphere thanks to its high rate of carbon capture, relatively low cost and moderate energy, even when compared to the best lab-based systems,” said Meenesh Singh, assistant professor of chemical engineering in the UIC College of Engineering and corresponding author on the paper.
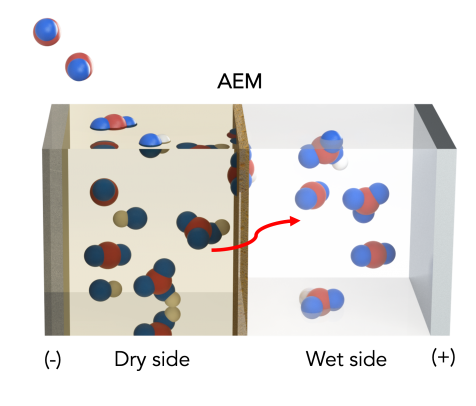
Using a previously reported theoretical concept, the scientists modified a standard artificial leaf system with inexpensive materials to include a water gradient — a dry side and a wet side — across an electrically charged membrane.
On the dry side, an organic solvent attaches to available carbon dioxide to produce a concentration of bicarbonate, or baking soda, on the membrane. As bicarbonate builds, these negatively charged ions are pulled across the membrane toward a positively charged electrode in a water-based solution on the membrane’s wet side. The liquid solution dissolves the bicarbonate back into carbon dioxide, so it can be released and harnessed for fuel or other uses.
The electrical charge is used to speed up the transfer of bicarbonate across the membrane.
When they tested the system, which is small enough to fit in a backpack, the UIC scientists found that it had a very high flux — a rate of carbon capture compared with the surface area required for the reactions — of 3.3 millimoles per hour per 4 square centimeters. This is more than 100 times better than other systems, even though only a moderate amount of electricity (0.4 KJ/hour) was needed to power the reaction, less than the amount of energy needed for a 1 watt LED lightbulb. They calculated the cost at $145 per ton of carbon dioxide, which is in line with recommendations from the Department of Energy that cost should not exceed around $200 per ton.
“It’s particularly exciting that this real-world application of an electrodialysis-driven artificial leaf had a high flux with a small, modular surface area,” Singh said. “This means that it has the potential to be stackable, the modules can be added or subtracted to more perfectly fit the need and affordably used in homes and classrooms, not just among profitable industrial organizations. A small module of the size of a home humidifier can remove greater than 1 kilogram of CO2 per day, and four industrial electrodialysis stacks can capture greater than 300 kilograms of CO2 per hour from flue gas.”
The UIC scientists report on the design of their artificial leaf and the results of their experiments in “Migration-assisted, moisture gradient process for ultrafast, continuous CO2 capture from dilute sources at ambient conditions,” which is published in Energy & Environmental Science.
The research is funded by a grant (DE-SC-0022321) from the U.S. Department of Energy. A patent application titled “Artificial photosynthetic systems for integrated carbon capture and conversion” has been filed by the Office of Technology Management at UIC.
Co-authors of the paper from UIC, Argonne National Laboratory, Oklahoma State University and Braskem are Aditya Prajapati, Rohan Sartape, Tomas Rojas, Naveen Dandu, Pratik Dhakal, Amey Thorat, Jiahan Xie, Ivan Bessa, Miguel Galante, Marcio Andrade, Robert Somich, Marcio Rebouças, Gus Hutras, Nathalia Diniz, Anh Ngo and Jindal Shah.
Categories
Topics
artificial leaf, carbon capture, carbon dioxide, chemical engineering, climate change, College of Engineering
