How protein disposal enzymes maintain metabolic health
Research at the University of Illinois Chicago may have deciphered a mysterious side effect of anti-cholesterol drugs by discovering how cells clear mitochondrial proteins.
Like keeping a clean house, cells must regularly clear out proteins by marking them for removal and destruction. The laboratory of Shafi Kuchay, assistant professor of biochemistry and molecular genetics at UIC, studies the family of enzymes that marks cellular proteins for degradation. Precise control of this protein disposal system is important for health; too much or not enough removal can cause dysfunction and disease.
In a paper published in Cell Reports, a team led by Kuchay describes how one of these enzymes is dispatched to the mitochondria — often called the metabolic powerhouses of the cell. Adding a lipid, or fat, molecule to the enzyme FBXO10 sends it to the outer layer of the mitochondrial membrane. There, the enzyme adds a molecular signal called ubiquitin to the proteins that are destined for degradation.
Experiments that disrupted this process increased the crowd of proteins in mitochondria and led to mitochondrial dysfunction and impaired metabolism. That can have serious repercussions; one experiment found that skeletal muscle cells don’t develop when the enzyme trafficking to the mitochondria is interrupted.
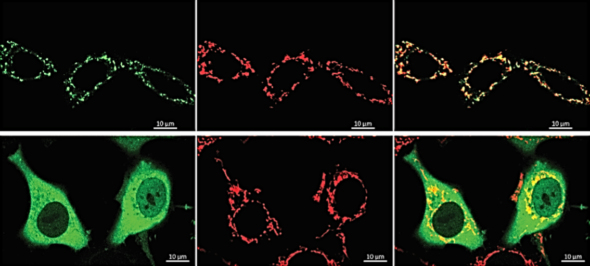
That may explain the muscle problems some people experience when taking statin drugs for high cholesterol, said Kuchay, who is also a member of the University of Illinois Cancer Center. Reducing cholesterol levels with statins could diminish the lipids needed to route FBXO10 for its custodial work in the mitochondria.
Conversely, the signals that route the FBX010 enzyme to mitochondria could be used to design new treatments that specifically target this cellular compartment and treat diseases caused by abnormal mitochondrial proteins.
“Mitochondria play a central role in metabolism. If there is an aberrant protein in the mitochondria that one would need to remove to improve metabolic health, we can utilize this enzyme to restrict the removal only to the mitochondria,” Kuchay said. “The advantage of precision targeting is to reduce side effects.”
Additional UIC researchers on the paper include Sameer Ahmed Bhat, Zahra Vasi, Liping Jiang, Shruthi Selvaraj, Rachel Ferguson, Sanaz Salarvand, Anish Gudur, Ritika Adhikari, Veronica Castillo and Hagar Ismail.
