UIC scientists contribute to first X-ray of single atom
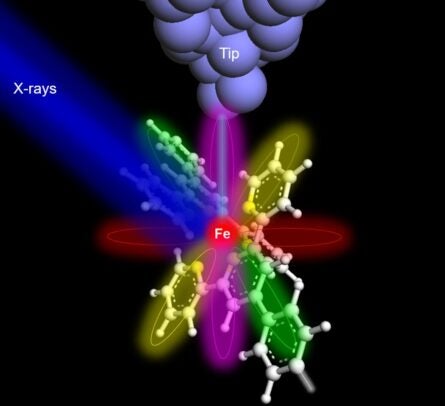
We’re used to X-rays in hospitals, dental offices and airports, where the 19th-century technology is still used to capture images of bones, teeth and other dense objects. But scientists also use X-rays to study the composition of samples and materials and examine very small targets in medicine, biology, physics and engineering.
In a new paper published in Nature, a multi-institutional team of scientists set a new standard for X-rays on the microscopic level, characterizing a single atom for the first time. The co-authors included Tomas Rojas and Anh Ngo of University of Illinois Chicago.
Previously, the smallest sample captured by an X-ray was 10,000 atoms. The research team designed a new synchrotron instrument located at the Advanced Photon Source of Argonne National Laboratory, using a sharp metal tip detector placed in extreme proximity to an iron or terbium atom.
The experiments produced X-ray absorption spectra — “fingerprints” that could be used to successfully identify the target atoms and their chemical properties. In the future, scientists could use the method to identify trace amounts of different elements in materials important for applications in quantum technology or in samples for medical or environmental research.
Read more about the study in a news release from Ohio University.
Categories
