A dynamic duo of cells identified in lung blood vessels
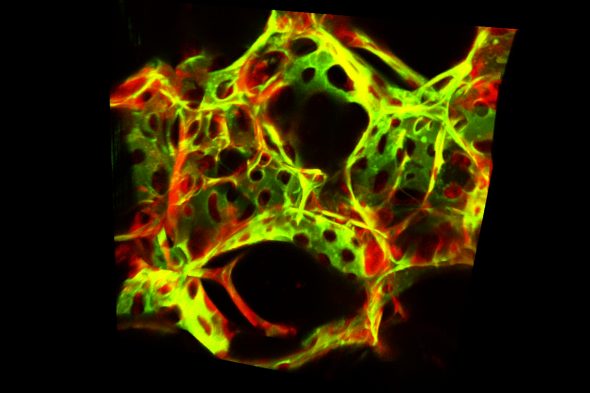
Scientists at the University of Illinois Chicago have analyzed gene expression data from more than 35,000 blood vessel cells from the lungs of mice and identified two subtypes.
One subtype, which they call immune endothelial cells, or immuneECs, preferentially expressed more genes involved in inflammation and the regulation of the immune response. The devEC subtype, for developmental endothelial cells, expressed more genes involved in cell development, like cell regeneration and proliferation.
The findings are published in the journal JCI Insight. They could lead to better treatments for lung infections, which can be dangerously exacerbated by unchecked inflammation.
The UIC team, led by Dr. Jalees Rehman, classified the subtypes by extracting lung tissue from mice engineered to express a fluorescent protein only in the blood vessel endothelial cells. Using a fluorescence cell sorter, the scientists isolated the lung endothelial cells. They then sequenced RNA from thousands of individual endothelial cells and categorized them based on their prominent gene signatures.
They found multiple groups of endothelial cells, with two dominant cell types — immuneECs and devECs — which changed over time during inflammation.
“Across our experiments, consistently we observed that the blood vessel cells of the lung seem to have these different functions and groupings, and the two predominant groups become even more distinct when responding to infection or stress,” said Rehman, UIC professor in the department of pharmacology and regenerative medicine and the department of medicine at the College of Medicine. “Importantly, we also analyzed publicly available datasets of human lungs and found similar distinct groups of endothelial cells as we had observed in the mouse lungs.”
In their experiments, Rehman and his colleagues studied cells from mice with healthy lungs and mice with lungs that were injured or fighting infections due to a bacterial toxin or influenza virus. While both subtypes were present in healthy and sick lungs, the gene expression profiles of each subtype further diverged in response to infections or injury.
As illness or injury progressed, immuneECs expressed more immune response genes, like major histocompatibility complex genes that act as a beacon for infection-fighting T-cells. As the lungs improved, devECs expressed more repair genes, like the vascular development gene Sox17, which promotes blood vessel growth and regeneration. Upon analyzing the RNA profiles of lung endothelial cells obtained from nonhuman primates with a SARS-CoV-2 infection, the researchers also found distinct groups of lung endothelial cells.

“Part of the blood vessel endothelial cells become highly inflammatory, and part of them retain that ability to regenerate,” Rehman said. “Our data also suggested that after some time, the developmental subtype, which is essentially primed to regenerate, gives rise to cells that start proliferating and regenerating the blood vessels that were damaged during the infection.”
The UIC scientists say understanding the teeter-tottering balance between the subtypes and exploiting this division of labor could lead to targeted treatments for conditions involving the lung blood vessels like acute respiratory distress syndrome, which occurs during severe lung infections. In these infections, immuneECs may play a key role in activating the initial immune response needed to fight off the pathogens. However, during severe infections, the immune system can overreact and start damaging its own lung tissue even when the virus or bacteria are cleared.
“This study tells us that if we want to treat the blood vessels of the lungs, we should specifically target the subtype that is out of balance. If the immune response is excessive as is often the case in severe COVID-19, it might be important to dampen the activity of immune regulatory endothelial cells while enhancing the growth of the regenerative endothelial cells,” Rehman said. “By understanding that the blood vessel cells have these different functions, we can work toward therapies that are more targeted to one type of blood vessel cell versus the other type of blood vessel cell.”
Rehman said the findings are a good reminder, too, that our lungs are always in contact with the outside world, and this may be why lung blood vessels need such a fine-tuned balance between endothelial cell subtypes, which can activate or amplify the immune response, whereas other endothelial cells can help repair the blood vessels after the infection starts subsiding.
“We’re always breathing in pathogens, unlike other organs such as the heart or the brain which are much more shielded from the pathogens of the outside world. That’s why that balance, this intricate system having both an inflammatory and a regenerative response, is so important in the lungs,” Rehman said.
The JCI Insight article, “Single-cell transcriptomic profiling of lung endothelial cells identifies dynamic inflammatory and regenerative subpopulations,” is co-authored by the UIC research team, which included Lianghui Zhang, Shang Gao, Zachary White, Yang Dai and Asrar Malik.
The research was supported by grants from the National Institutes of Health (R01HL157489, P01HL060678, P01HL151327, R01HL90152 and R01HL152515) and the American Heart Association (18CDA34110068).
