Getting the most therapeutic potential out of cells
A simple change in the way donor cells are processed can maximize a single cell’s production of extracellular vesicles, which are small nanoparticles naturally secreted by cells, according to new research from researchers at the University of Illinois Chicago.
The finding offers new avenues for research around cellular therapies, which use transplanted cells — like stem cells or immune cells, either from the patient or a donor — to help the body heal or work better, and patients and their doctors want the most bang for their buck in terms of potency. For injuries in the lungs, like those caused by acute respiratory distress syndrome, treatments that use extracellular vesicles have shown promise, but remain expensive and limited by the number of donated cells needed to reach a therapeutic level.
The researchers, led by Jae-Won Shin, have been studying how extracellular vesicles work. Through experiments, they found that altering the material in which the donor cells are processed can have a strong impact on the potency of extracellular vesicles.
“We were very surprised that a simple environmental change could have such a significant impact,” said Shin, UIC assistant professor in the department of pharmacology and regenerative medicine and the department of biomedical engineering. “This tells us that cells interact differently in different tissues, and this impacts how they secrete extracellular vesicles and influence other cells around them.”
The key, they found, was using a soft hydrogel material that more closely resembles the natural environment of tissues to prepare the particles. When they compared the particles cultured from cells in traditional materials with those cultured in a softer material, they saw that the extracellular vesicles were secreted in a greater quantity in the softer substrate.
“In the stiff substrates, cytoskeletal structures in cells are dense and less flexible. This makes it difficult for extracellular vesicles to exit the cells. But in the soft substrate, these structures are less dense, more bendable and more spread out, making the environment more conducive to the secretion of the particles by cells,” said first author Stephen Lenzini, a UIC alumnus who worked on the study in Shin’s lab as a graduate student.
Shin said, “That’s why fewer donor cells are needed to produce the same number of particles.”
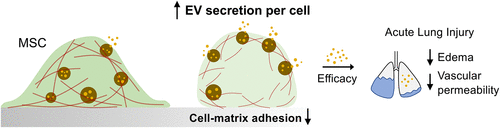
In the stiff substrates (dark green), cytoskeletal structures are dense and inflexible (red rods). This makes it difficult for extracellular vesicles (orange dots) to exit bodies (brown circles). In soft substrates (light green), these structures are shorter, bendable and more spread out, making the cell much more porous and conducive to the secretion of the particles, which can potentially be used to heal damaged lungs. (Image: Lenzini, et al.)
They also compared the therapeutic potential of the particles produced in the different materials. They observed that the same dose of extracellular vesicles produced from the softer substrate was much more effective at facilitating repair processes than the extracellular vesicles produced from a traditional harder substrate.
“Understanding this opens the door for many new avenues of investigation for lab and clinical trials of treatments that use donor extracellular vesicles to repair damaged tissues, like which occurs in the lungs of some COVID-19 patients who face complications like ARDS,” he said.
Additional co-authors of the study, which is titled “Cell–Matrix Interactions Regulate Functional Extracellular Vesicle Secretion from Mesenchymal Stromal Cells” and is published in ACS Nano, are Koushik Debnath, Jagdish Joshi, Sing Wan Wong, Kriti Srivastava, Xue Geng, Ik Sung Cho, Angela Song, Raymond Bargi, James C. Lee, Gary C.H. Mo, and Dolly Mehta, all of UIC.
