Molecule manages blood vessel permeability in inflammation
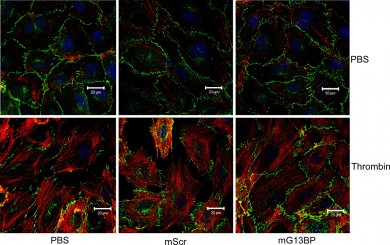
Green adherens junctions appear discontinued in the bottom left and middle images, which were treated with an inflammatory agent. Adherens junctions are still well-organized with the addition of a Galpha 13 blocker after exposure to the same inflammatory agent, as seen on bottom right. Photo by Haixia Gong.
Researchers at the University of Illinois at Chicago describe how a protein involved in cell differentiation and migration also plays a crucial role in controlling the passage of materials out of the blood stream. Their results were published online March 3 in The Journal of Experimental Medicine.
Loss of control of the movement of materials across the blood vessel wall is one of the hallmarks of chronic inflammatory and vascular diseases including acute lung injury, atherosclerosis and hypertension. Cancer cells take advantage of compromised endothelial cells that line blood vessels when the cancer cells metastasize and enter the bloodstream to travel to other tissues and organs.
“By better understanding the general principles behind how the endothelial layer works, we might be able to fine-tune it so that it works better in people where it is compromised or too leaky,” says Asrar B. Malik, Schweppe Family Distinguished Professor and head of pharmacology in the UIC College of Medicine, one of the authors of the paper.
Blood vessels are lined by a single layer of endothelial cells that form a selective barrier and manage the passage of nutrients, proteins and cells in and out of the blood stream. The integrity of the endothelium depends on structures called adherens junctions that bind the endothelial cells to each other to form a barrier.
During an inflammatory response, adherens junctions are disassembled and the endothelial layer becomes leaky, allowing immune cells to move out of the bloodstream to sites of infection. But during acute or chronic inflammation, the endothelial barrier may remain permeable longer than is beneficial, and can become a factor in many diseases.
“We wanted to look at what governs the assembly and disassembly of adherens junctions as a first step in the development of interventions that could reduce endothelial leakiness,” Malik said.
Malik and colleagues discovered that a signaling protein called Galpha 13 was involved in the assembly and disassembly of adherens junctions. Human lung endothelial cells with reduced levels of Galpha 13 showed less disruption of the barrier in response to inflammation than cells with normal levels of Galpha 13.
In an animal model, the researchers showed that the absence of Galpha 13 made adherens junctions more resistant to disruption in response to inflammation — and could reduce mortality from sepsis.
“This finding opens up the possibility for reducing the inappropriate trafficking of blood components and cancer cells across the endothelial layer,” Malik said. “Galpha 13 offers a promising new target for therapies for chronic inflammatory and vascular diseases.”
Research assistant professors Haixia Gong and Xiaopei Gao, doctoral students Shaoting Feng and Alexander Garcia, post-doctoral fellow M. Rizwan Siddiqui, assistant professor Yulia Komarova, research associate professor Stephen Vogel and associate professor Dolly Mehta, all of the UIC pharmacology department, and Marcelo Bonini, UIC assistant professor of medicine and pharmacology, are also authors on the paper.
This research was supported by grants R01 HL045638 and P01 HL060678 from the National Institutes of Health.
