Protein receptor in endothelial cells promotes lung healing
In the lung’s blood vessels, endothelial cells form a protective barrier that regulates how fluids and other substances, like white blood cells, are transported in and out of lung tissue through the bloodstream. However, in some cases, the endothelium becomes more permeable — it becomes “leaky,” allowing for increased inflammation and fluid buildup in the lungs, a condition known as lung edema. These are classic signs of respiratory conditions like acute respiratory distress syndrome, or ARDS, for which treatment options are limited both in number and in their ability to restore lung vascular endothelial barrier function.
Now, researchers at the University of Illinois Chicago have identified a receptor expressed inside some endothelial cells that, when activated, helps to promote tissue regeneration and self-repair in mouse models of ARDS. Their findings are reported in Circulation Research, a journal of the American Heart Association.
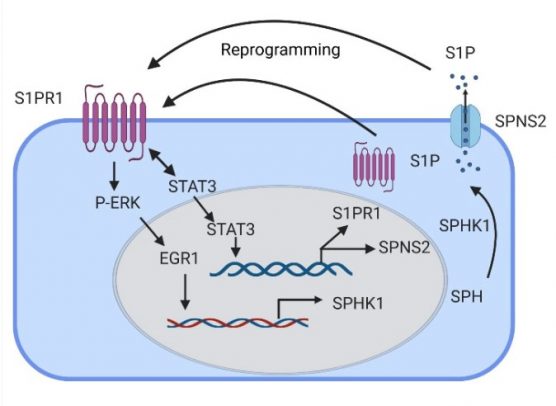
“In our paper, we described a previously unexplored endothelial cell population in the lungs which express sphingosine 1 phosphate receptor 1 with the capacity to repair endothelial integrity, which may prevent inflammatory lung vascular injury,” said Dolly Mehta, UIC professor in the department of pharmacology and regenerative medicine at the College of Medicine and corresponding author of the study.
Sphingosine 1 phosphate receptor 1, or S1PR1, belongs to a family of receptors that pair up with G-proteins and play a role in developing and maintaining blood vessels in several organs, including lungs.
For the study, the researchers observed mice with leaky lungs and low-grade inflammation.
First, they studied the generation of endothelial cells expressing green fluorescent protein (GFP)-S1PR1 in the mouse models. In these mice, any increase in GFP was an indication of S1PR1 activity. They also found that by activating certain cell signals called transcription factors, they could increase the production of sphingosine 1 phosphate and help the cell to generate more S1PR1-positive endothelial cells.
The researchers then studied the effects of the activated receptor in rescuing lung vascular homeostasis. They injected endothelial cells expressing GFP-S1PR1 into mice with leaky lungs and found it reprogramed endothelial cells by turning on a self-healing signal. The mice who received the injection showed more signs of a healthy and selective endothelial barrier, such as reduced fluid buildup.
“Our findings show that cultivation of endothelial cells that express S1PR1 activates the endothelial regenerative program to mediate endothelial repair. These results raise the possibility of harnessing this pathway for potential drug treatments to restore vascular homeostasis in inflammatory vascular injury states,” Mehta said.
Co-authors of the study are Md-Zahid Akhter, Jagdish Joshi, Vijay Avin Balaji Ragunathrao, Mark Maienschein-Cline, and Asrar Malik of UIC and Richard Proia of the National Institute of Diabetes and Digestive and Kidney Diseases.
This work was supported by grants from the National Institutes of Health (HL060678, HL137169, HL084153), the American Heart Association (19POST34450241), and the National Center for Advancing Translational Sciences (UL1TR002003).
Categories
Health Sciences Colleges, Research
Topics
ARDS, blood vessels, College of Medicine, lungs, Pharmacology and Regenerative Medicine, research
