Researchers describe how microtubules assemble, disassemble for the first time
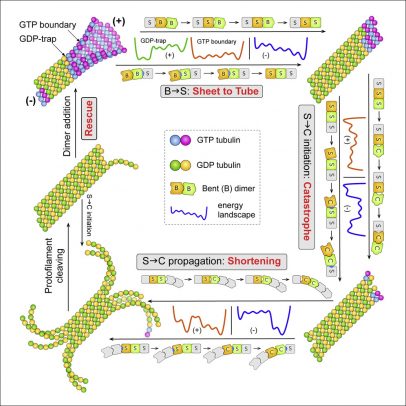
University of Illinois Chicago researchers describe for the first time the processes behind the assembly and disassembly of microtubules. The discovery, led by Ao Ma, associate professor of bioengineering at the UIC College of Engineering, is reported in the journal Cell Systems.
Microtubules are made of small subunits of proteins called tubulin and provide structural support to cells as well as pathways used to transport materials in the cell. They also are crucial in cell division.
Microtubules can assemble and disassemble very rapidly, allowing flexibility in their function within the cell, but researchers long have puzzled over exactly how this process occurs.
Ma and colleagues have deduced that microtubules’ ability to assemble and disassemble so quickly is because of small subunits that make them up can conform to different shapes. By undergoing these conformational changes, they can rapidly dissolve or form. When the researchers created a model based on their theory and tested it against real-world data, the model held up.
“Based on experimental data in the literature on the different structures of tubulin subunits and using knowledge from physics and chemistry, we inferred all the steps and conditions for the structural changes of tubulin subunits and developed a comprehensive computational model on how these changes happen in microtubules,” Ma said. “Our model was able to reproduce all the experimental observations of the assembly and disassembly processes for purified microtubules, providing a satisfying answer to the 30-year-old question of how microtubules assemble and disassemble.”
The findings help provide a new foundation and guiding framework for developing new drugs and therapeutic strategies for microtubule-related diseases that interfere with microtubule assembly dynamics including some cancers, neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease and amyotrophic lateral sclerosis and neurodevelopmental disorders including autism spectrum disorders.
“One exciting new direction for anticancer therapy involves proteins that regulate microtubule assembly dynamics in cells,” Ma said. “Our findings provide a foundation for understanding how these proteins work, enabling the design of drugs that target these crucial proteins.”
UIC’s Shannon Stewman and Kenneth Tsui Chicago are co-authors on the paper.
Written by Sharon Parmet
