Role of key metabolic enzyme in liver fibrosis defined by UIC scientists
Liver fibrosis is an irreversible response of the organ to injury and illness that leads to cirrhosis and failure; its only treatment is an organ transplant. New research from the laboratory of Nissim Hay, distinguished professor of biochemistry and molecular genetics at UIC, identified a key pathway that triggers this unwelcome defense, suggesting potential drug targets for fibrosis as well as cancer.
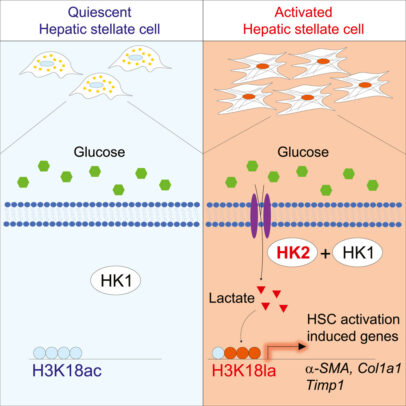
The study, published in Cell Metabolism, zeroed in on the enzyme hexokinase-2, which executes the first committed step in the conversion of glucose into energy. This glycolysis pathway, one of the cornerstones of biology, can grow overactive in both cancer cells and the hepatic stellate cells that set off liver fibrosis.
Previously, Hay’s group found that hexokinase-2 was elevated in cancer cells, a possible reason for their dysregulated glucose metabolism. Could the same be true for the liver, and if so, how does this enzyme induce the process of fibrosis?
In mice where hexokinase-2 was genetically deleted from the hepatic stellate cells, fibrosis was inhibited, confirming a key role for the enzyme. And further experiments identified an unlikely intermediary for its effects: lactate.
Once considered a mere waste product of glucose metabolism — as in the lactic acid produced in muscles during exercise — lactate recently has been found to play an interesting role in the regulation of gene expression inside cells. Lactate also has been previously implicated in promoting liver fibrosis, but the mechanism remained elusive.
Hay’s group, led by graduated PhD student Hyunsoo Rho, determined that elevated lactate in hepatic stellate cells causes lactylation of histones, the structure that DNA wraps around. This modification switches on a group of genes that activate these cells and induce fibrosis, the scientists found. Even in the mice without hexokinase-2, increasing lactate could switch on this pathway, further confirming the mechanism and that the enzyme is a promising drug target.
“This is a potential therapy, because the systemic deletion of hexokinase-2 does not have any significant physiological impact, but it can inhibit cancer and also can inhibit fibrosis,” Hay said. “We have provided the proof of concept, and now the challenge is to find a selective inhibitor.”
The paper, “Hexokinase 2-mediated gene expression via histone lactylation is required for hepatic stellate cell activation and liver fibrosis,” was published online in Cell Metabolism on July 17. Additional UIC co-authors include Alexander R. Terry and Constantinos Chronis, both of the UIC Department of Biochemistry and Molecular Genetics.