Study unveils new mechanism for long-distance cell communication
An extracellular vesicle — a nanoparticle released by cells — can use jerky movements similar to a car weaving in and out of traffic to navigate the obstacle-filled environment outside of cells, according to new discoveries made by researchers at the University of Illinois at Chicago.
Their findings, published in Nature Nanotechnology, are a key first step to efficiently use extracellular vesicles, or EVs, as a therapeutic that targets diseases, such as lung injury and cancer.
“Although EVs were discovered over 30 years ago, many believed that EVs were cellular junk that was trapped in the extracellular matrix,” said senior author Jae-Won Shin, UIC assistant professor of pharmacology and bioengineering at the College of Medicine. “Within the last 10 years, the field has learned that EVs aren’t junk. They play a critical role in sending signals for long-distance communication between cells.”
The extracellular matrix is a gel-like net of compacted protein chains and sugars that surrounds cells. To understand how billions of EVs navigate through the matrix, Shin’s lab used improved imaging, vesicle labeling and motion capturing technologies that weren’t available decades ago.
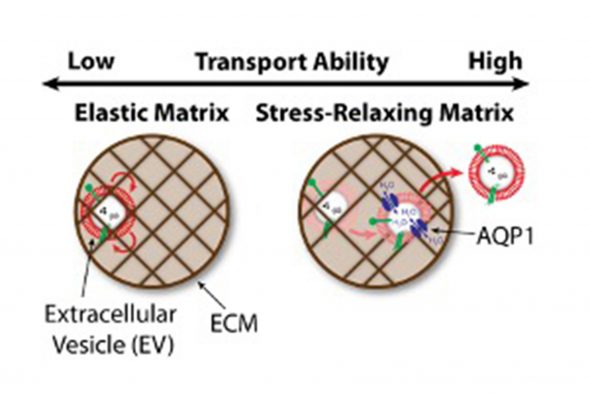
“We saw that the gaps in the matrix were smaller than the size of EVs and thought travel would be difficult,” Shin said. “It was a surprise when we observed that the EVs traveled much more readily than we thought in certain conditions.”
The researchers utilized an artificial matrix, called a hydrogel, to study whether its structure played a role in EV navigation. They customize the hydrogel’s stiffness and how well the hydrogel could relax after being stressed by an object in order to make the hydrogel more or less like the matrix in the body.
“The EVs became stuck when the hydrogel couldn’t relax over time, like rubber,” said Stephen Lenzini, first author and UIC graduate student in the College of Engineering. “The hydrogel needed to have a stiff backbone to provide some sort of structure, but after a stress it also had to relax enough to rearrange itself over time, which allowed the EVs to move around. The interesting finding was that this ability to move that occurred for EVs in some materials did not occur for synthetic particles of similar size.”
The same membrane that EVs use to protect their cargo also was essential for its own flexibility in tight spaces. When aquaporin-1 — a membrane protein that allows water in and out of EVs — stopped functioning, the EVs became stuck. The permeation of water through aquaporin-1 in the membrane was essential for EVs to slip through the hydrogel gaps.
“This study has opened new avenues into studying the distribution of EVs and their contents through tissues,” Lenzini said.
The findings advance the UIC research group closer to engineering effective delivery systems, according to Shin.
“There are a range of diseases that undergo substantial changes in their environment. In fibrosis and some cancers, the tissues and matrix become more rigid as time progresses. In some cancers, the distribution of EVs have led to disease spreading,” he said. “So, understanding how EVs are dispersed is critical for developing these cell-free therapeutics and stopping disease progression.”
Raymond Bargi and Gina Chung are co-authors on the paper.
This work was supported by grants from the National Institutes of Health (R01HL141255, R00HL125884 and T32HL07829) and the American Heart Association (19PRE34380087).
Written by Natasha Wadlington
