UIC chemical engineers first to functionalize boron nitride with other nanosystems
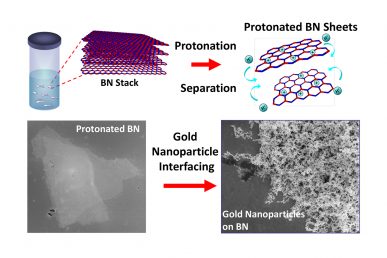
Treatment with a superacid causes boron nitride layers to separate and become positively charged, allowing for it to interface with other nanoparticles, like gold. (Credit: Berry, et al).
Researchers at the University of Illinois at Chicago have discovered a route to alter boron nitride, a layered 2D material, so that it can bind to other materials, like those found in electronics, biosensors and airplanes, for example. Being able to better-incorporate boron nitride into these components could help dramatically improve their performance.
The scientific community has long been interested in boron nitride because of its unique properties —it is strong, ultrathin, transparent, insulating, lightweight and thermally conductive — which, in theory, makes it a perfect material for use by engineers in a wide variety of applications. However, boron nitride’s natural resistance to chemicals and lack of surface-level molecular binding sites have made it difficult for the material to interface with other materials used in these applications.
UIC’s Vikas Berry and his colleagues are the first to report that treatment with a superacid causes boron nitride layers to separate into atomically thick sheets, while creating binding sites on the surface of these sheets that provide opportunities to interface with nanoparticles, molecules and other 2D nanomaterials, like graphene. This includes nanotechnologies that use boron nitride to insulate nano-circuits.
Their findings are published in ACS Nano, a journal of the American Chemical Society.
“Boron nitride is like a stack of highly sticky papers in a ream, and by treating this ream with chlorosulfonic acid, we introduced positive charges on the boron nitride layers that caused the sheets to repel each other and separate,” said Berry, associate professor and head of chemical engineering at the UIC College of Engineering and corresponding author on the paper.
Berry said that “like magnets of the same polarity,” these positively charged boron nitride sheets repel one another.
“We showed that the positive charges on the surfaces of the separated boron nitride sheets make it more chemically active,” Berry said. “The protonation — the addition of positive charges to atoms — of internal and edge nitrogen atoms creates a scaffold to which other materials can bind.”
Berry said that the opportunities for boron nitride to improve composite materials in next-generation applications are vast.
“Boron and nitrogen are on the left and the right of carbon on the periodic table and therefore, boron-nitride is isostructural and isoelectronic to carbon-based graphene, which is considered a ‘wonder material,'” Berry said. This means these two materials are similar in their atomic crystal structure (isostructural) and their overall electron density (isoelectric), he said.
“We can potentially use this material in all kinds of electronics, like optoelectronic and piezoelectric devices, and in many other applications, from solar-cell passivation layers, which function as filters to absorb only certain types of light, to medical diagnostic devices,” Berry said.
Co-authors on the study are Sanjay Behura of UIC; Kabeer Jasuja of the Indian Institute of Technology; and Kayum Ayinde, Christina Wilson, Myles Ikenberry, Keith Hon and David Moore of Kansas State University.
Categories
Topics
boron nitride, chemical engineering, College of Engineering, composite materials, superacid

