Miniature ‘tumor farm’ enables personalized drug screening for lung cancer
To fulfill the promise of personalized cancer treatment, clinicians need a system to rigorously and rapidly test cancer drugs against each patient’s tumor to determine their most effective therapy. A new model created at the University of Illinois Chicago could make it possible to perform this individualized screening process in the laboratory, revolutionizing the treatment strategy for lung cancer patients.
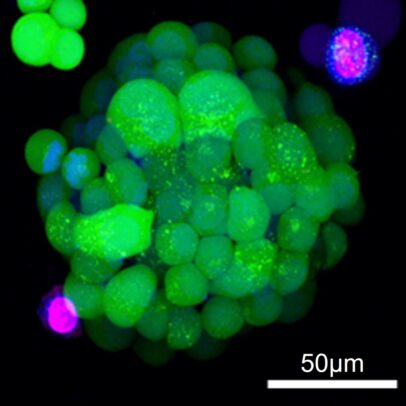
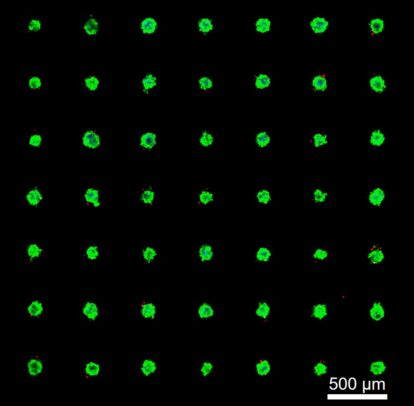
The platform, the result of a collaboration led by Ian Papautsky and Takeshi Shimamura, grows hundreds of microscopic tumors from cells harvested during a patient’s biopsy. Researchers can then expose these three-dimensional “organoids” to various drugs using microfluidics to predict which would be most effective for each patient in the clinic.
Preliminary studies published in the journal Lab Chip demonstrate that the system can accurately simulate lung tumors and elements of their microenvironment to evaluate drug sensitivity and resistance. If clinically approved, researchers could use the platform to quickly test a broad menu of drugs both alone and in combination for each individual patient — essentially a personalized, miniaturized large-scale trial.
“It’s really a perfect system for bringing science to the bedside,” said Shimamura, associate professor of surgery in the College of Medicine. “It’s a bridge between the patient and the research.”
Traditionally, researchers use tumor cell cultures for drug testing in the laboratory. These cells are grown on the surface of plastic plates that are very unlike their natural environment. By contrast, patient-derived organoids form a three-dimensional structure that more accurately reflects the shape and composition of the original tumor.
“These models are more representative of what’s going on in a patient,” said Papautsky, the Richard and Loan Hill Professor of biomedical engineering in the colleges of engineering and medicine. “When we did the drug screening on cells in two-dimensional culture and then used the same cells to build a three-dimensional structure, the drug tolerance and response were very different.”
Papautsky and Shimamura’s collaboration set out to shrink those organoids down to a size that would allow scientists to run hundreds of such tests simultaneously. They designed microwells — very small, U-shaped pockets where cells could form into organoids less than a quarter of a millimeter across. Hundreds of these microwells can fit inside a single well of a standard 48-well laboratory plate.
Using the Papautsky group’s expertise in microfluidics, the researchers designed a system capable of delivering drugs and biological factors to subpopulations among these tumors. The result is a panel of hundreds of nearly identical tiny tumors, sourced from a single patient, for large-scale drug screening.
“In the long run, we would obtain these samples from the clinic, isolate the tumors, put these cells straight into our device and create a drug response platform,” Shimamura said. “Then we may be able to offer a quick prediction of what might be a good combination to try on the patient.”
After introducing the model in a 2022 paper, the team continued to refine its features to better simulate the environment surrounding a tumor in a patient’s lungs. The new Lab on a Chip paper added a second type of cell, called cancer-associated fibroblasts, to the organoid system and replicated drug resistance effects observed in patients that couldn’t be explained by tumor cells alone.
Using this more realistic setup, researchers can develop new strategies to evade drug resistance that are more likely to translate into successful clinical use. The team also believes the platform could be adapted for studying other types of cancer and cellular diseases.
The researchers credit the support of the biomedical engineering and surgery departments and the interdisciplinary environment at UIC for making such a promising new technology possible.
“One of the reasons I came to UIC is because the university offers diversity in disciplines, with a particular strength in bioengineering,” Shimamura said. “That enables us to really work across disciplines, and that is extremely important for this type of challenge.”
Other UIC co-authors on the new paper include lead author Qiyue Luan, as well as Ines Pulido, Angelique Isaguirre and Jian Zhou. Funding for the research was provided by the U.S. Department of Defense and the National Cancer Institute.