UIC to lead national COPD clinical trial
The University of Illinois at Chicago will lead a $14.6 million, multi-center research project to determine which of two drugs — azithromycin, an antibiotic, or roflumilast, an anti-inflammatory medication — is the most effective at treating chronic obstructive pulmonary disease, which is also known as COPD.
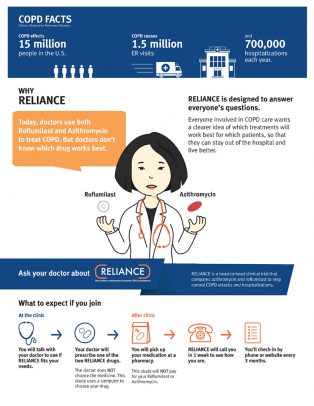
COPD, an umbrella term for a number of lung conditions that make it difficult for a person to breathe, affects 15 million people living in the U.S. and is a leading cause of hospitalizations and death in the country.
Currently, doctors prescribe either azithromycin or roflumilast for months or years for patients with COPD to reduce the risk of flare-ups, known as COPD exacerbations, which primarily are caused by smoking.
While the two drugs have both been shown to help prevent COPD exacerbations, doctors do not know which drug works best for which patient.
“Previous studies of these medications have been about finding out if they work compared to a placebo pill in highly selected patients and study environments,” said trial principal investigator Dr. Jerry Krishnan,
associate vice chancellor for population health sciences and professor of medicine and public health at UIC. “This is the first study to test these medications as they are used in routine health care settings.”
The clinical trial, known as the RELIANCE study, is designed by researchers with the help of patients, caregivers, professional societies and the COPD Foundation, the largest patient advocacy organization for COPD in the U.S.
It will follow more than 3,000 patients with COPD for up to three years. Patients will be enrolled at their doctor’s office as they receive their usual COPD care at more than 30 centers across the U.S.
The researchers will track participants’ health by telephone and by monitoring their electronic health records and medical and pharmacy claims information. The study will help to determine which medication is more effective in reducing the risk of urgent care and emergency room visits, hospitalizations and death. The researchers also will track side effects and indicators of physical and mental health.
“The RELIANCE study will compare the two medications head-to-head and determine which is best to reduce the risk of death and hospitalization in people with COPD,” Krishnan said. “Specifically, we want to find out which medication works best for people who are current smokers and for people who are former smokers.”
In addition, the researchers will compare the drugs according to gender, comorbid conditions and other patient characteristics.
“Patients with COPD deserve an individualized treatment plan. The RELIANCE study will help us to do that,” Krishnan said.
Other RELIANCE investigators at UIC include Dr. Valentin Prieto-Centurion, assistant professor of medicine, and Kim Erwin, co-founder and co-director of the Institute for Healthcare Delivery Design and research professor of design.
Erwin says that RELIANCE is the first clinical trial to integrate a dedicated communication center, staffed by human-centered design experts, to create patient-focused recruitment and retention strategies.
“We have successfully engaged COPD patients to design a novel consent form that is easy to read and understand. This is a rarity in clinical trials,” she said. “We are extending that design strategy to rethink all trial materials, so that everyone involved — patients, families, doctors and study staff — proceed with a clear understanding of RELIANCE, how it works and what’s being asked of them.
“We are also focused on making this a ‘learning trial’ for patients, who tell us that they want to be considered partners and included in trial updates and discoveries as they emerge. To have a communication center dedicated to addressing the real-world needs of diverse stakeholders is another unusual feature of RELIANCE,” Erwin said.
The leadership team for the study, also called the steering committee, includes Krishnan, Erwin and researchers at Johns Hopkins University, the COPD Foundation, Kaiser Permanente Northwest, the University of Nebraska, the University of Colorado Denver, and the U.S. Food and Drug Administration. UIC will lead the project and serve as the clinical coordination center. Johns Hopkins will manage the data coordinating center.
The clinical trial is funded by the Patient-Centered Outcomes Research Institute (PCS-1504-30430).
The clinical centers involved in the trial include Baylor Scott and White Health-North, Baystate Health, Cleveland Clinic, Denver Health and Hospital Authority, Duke University, Henry Ford Health System, Johns Hopkins University, Kaiser Permanente Northwest, Loyola University Medical Center, Medical College of Wisconsin, Medical University of South Carolina, Mount Sinai Health System Network, NorthShore University HealthSystem, Northwestern University, Ochsner Health New Orleans, Ohio State University, Providence Health and Services Washington, Temple University, University Hospitals Cleveland Medical Center, University of Alabama Birmingham, University of Arizona, University of Iowa, University of Michigan, University of Missouri Truman Medical Center, University of North Carolina, University of Pittsburgh Medical Center, University of Vermont, Washington University and UI Health, UIC’s academic medical center and network of community-based clinics.
Categories
Health Sciences Colleges, Patient Care, Research, UI Hospital
Topics
clinical trials, COPD, East Meets West, lung disease, research